Heteronuclear spin-spin couplings between nuclei such as 1H and 13C in NMR spectroscopy provide a wealth of chemical structural information. Sometimes, however, the problem can be that the information is too rich and adorned with competing information such as 1H-1H scalar couplings. A variety of very elegant and interesting NMR methods have been developed over the years to simplify complex NMR spectra and facilitate the measurement of coupling constants. Over the next few posts I will attempt to provide a brief overview of some of most important and widely used methods. In this first post, the use of selective decoupling in high-resolution 13C spectra is shown as a first, and in many ways the simplest, experimental approach. The data shown below were collected on a 3-channel JEOL ECZ-500 spectrometer equipped with a ROYAL-HFX probe and were processed using JASON software.
Double resonance methods
The idea of double-resonance NMR, aka decoupling, arose at the dawn of NMR in a thesis by Weston A. Anderson. For an excellent overview putting Wes’s work into perspective and of the time when chemists first “discovered” NMR, the blog by Ray Freeman about the early 1950’s, http://ray-freeman.org/nmr-history.html, is both illuminating, historically significant, and highly entertaining.
Perfluorinated molecule example
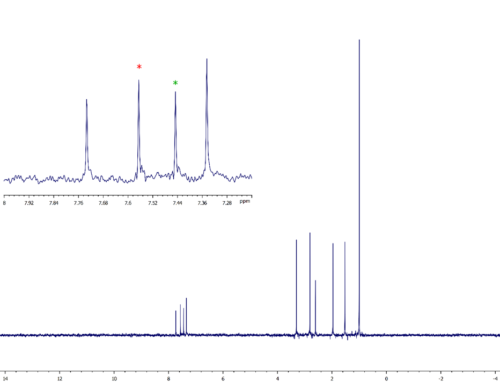
Let’s illustrate the application of double-resonance methods to the measurement of coupling constants using a simple perfluorinated molecule. The question to be answered is “what are the values of the many observable 19F-13C coupling constants from F2, F5, and F6 to each of the carbons?”. To answer that question is incredibly easy! First, acquire three 13C datasets each with selective 19F decoupling applied on either F2, F5, or F6. Then, compare the spectra with the fully 19F-coupled 13C spectrum and construct a table with the desired coupling constant information.

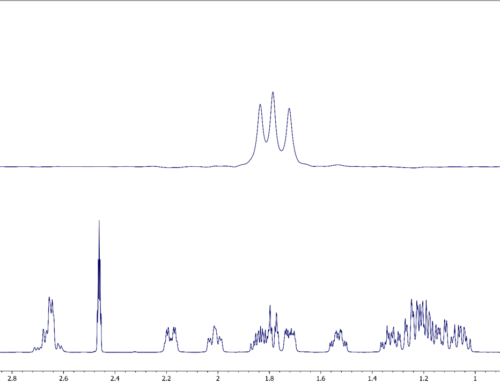
By creating simple expansions centered on each carbon resonance, all couplings are quite clear and easy to extract. As examples, two expansions will be shown; one for the C-5 with a directly attached fluorine and one for the quaternary carbon C-5.


Finally, we show a table with each of the 19F-13C couplings and all assignments for the example molecule.

Conclusions
In my first post on techniques for measuring coupling constants in NMR spectra, I have shown that by revisiting ideas born at the very beginning phases of NMR science, the pioneers at that time provided guidance to experimentally simple yet highly effective strategies to answering complex problems.
Want to learn more?
Why not try JEOL JASON software for yourself and discover how its intuitive user interface and advanced data processing and analysis tools can enhance your productivity. If you’d like know more about the new-generation JEOL ECZ Luminous NMR spectrometers and JEOL liquid-state NMR probes, just click the links.